Hybrid Theory Worksheet
Back to the other Bond Theory Chemistry Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Solutions Manual
- Consider CH4
- What orbitals are available for bonding for each atom involved in the molecule?
- Based on this would you expect all the C-H bonds in methane (CH4) to be identical?
- What have experiments shown?
- What does this indicate?
- This means that when an atom patricpiates in a bond, _____________
__________________________________________________________.
- How does the energy of the hybrids compare to the atomic orbitals?
- The number of hybrid orbitals formed is equal to _________________.
- What should you do first, when trying to determine the type of hybrid orbitals needed?
- Why?
- How many areas of electron density do the following account for?
- Lone Pairs
- Single bond
- Double Bond
- Triple Bond
- Fill In the Following
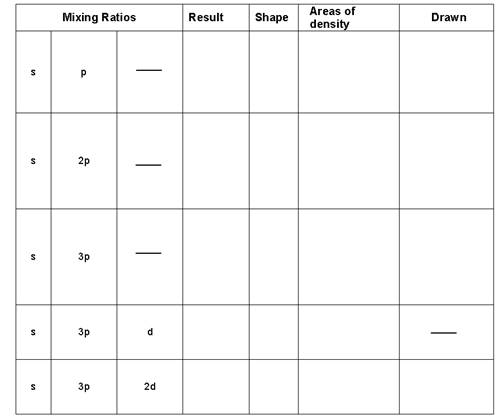
- Draw the following
- CH4
- CH2O
- What are sigma and pi bonds?
- What are each type of bond composed of?
- Predict the shape, hybridization of central atom and polarity for the following
- OF2
- TeF4
- BF3
- Label the hybridization of C, O, and N in the following molecules. Also count total number of sigma bonds and total number of pi bonds.

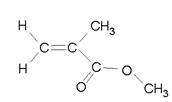
- Are all the atoms in the same plane?
- C2H2
- CH2CCH2
- How can CO32- help us understand the short falls of LE model?
